Voorblad
Indicatieaanvraag/studie:
Gemcitabine/Nabpaclitaxel na FOLFIRINOX bij gemetastaseerd pancreascarcinoom
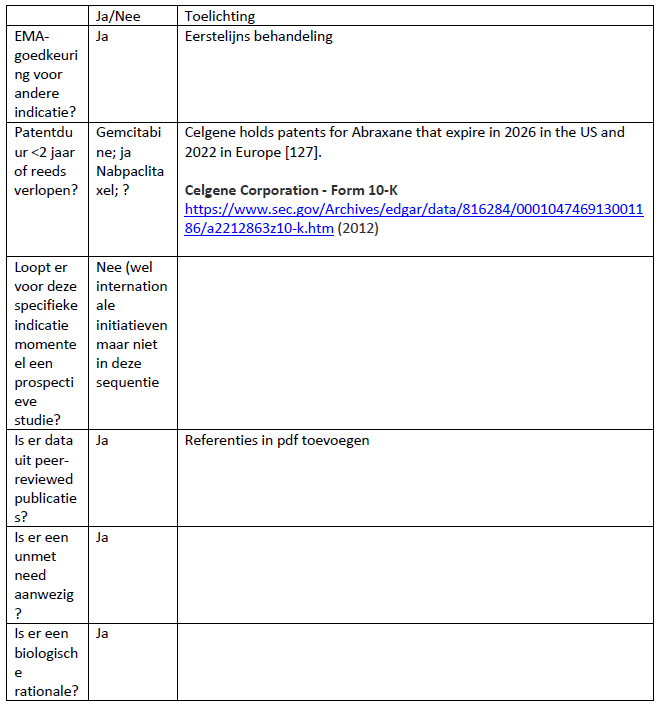
Beoordeling door CieOOM mogelijk: ja
Beoordeling voorleggen aan CieBOM: /nee
Is er naar oordeel cieOOM een gerandomiseerde studie mogelijk: ja
Waar hoort het thuis:
- Lijnverschuiving
Wijze van beoordelen (NRS of niet): ja
Huidige standaard: BSC
Huidige indicatietekst:
Lijn-/indicatieverschuiving
Tumortype, behandellijn en argumenten voor lijn-/indicatieverschuiving
Gemetastaseerd pancreascarcinoom waarvoor na FOLFIRINOX geen standaard opties zijn gedefinieerd
Medicament/middelen
Gemcitabine icm Nabpaclitaxel
Indicatie (EMA) en huidige indicatietekst (op farmatec)
Abraxane is used to treat the following cancers in adults:
- metastatic breast cancer, when the first treatment has stopped working and standard treatment including an ‘anthracycline’ (another type of cancer medicine) is not suitable. ‘Metastatic’ means that the cancer has spread to other parts of the body.
- metastatic adenocarcinoma of the pancreas, as a first treatment in combination with another cancer medicine, gemcitabine.
- non-small cell lung cancer, as a first treatment in combination with the cancer medicine carboplatin when the patient cannot have surgery or radiotherapy.
Korte bespreking van de studie(s)
Portal et al.;
From February 2013 to July 2014, all consecutive patients treated with A+G for histologically proven MPA after FOLFIRINOX-failure were prospectively enrolled in 12 French centres. A+G was delivered as described in the MPACT-trial, until disease progression, patient refusal or unacceptable toxicity.
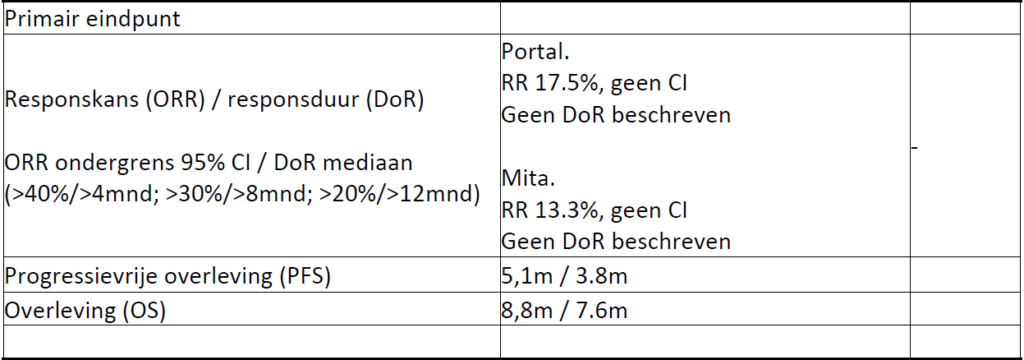
Fifty-seven patients were treated with Nab-paclitaxel plus gemcitabine, for a median of 4 cycles (range 1-12). The disease control rate was 58%, with a 17.5% objective response rate. Median overall survival (OS) was 8.8 months (95% CI: 6.2-9.7) and median progression-free survival was 5.1 months (95% CI: 3.2-6.2). Since the start of first-line chemotherapy, median OS was 18 months (95% CI: 16-21). No toxic deaths occurred. Grade 3-4 toxicities were reported in 40% of patients, consisting of neutropenia (12.5%), neurotoxicity (12.5%), asthenia (9%) and thrombocytopenia (6.5%).
Mita et al.;
This study was a multicenter prospective phase II study evaluating second-line GnP in patients with APC after failed first-line FX. The primary endpoint was response rate (RR), and the secondary endpoints were overall survival (OS), progression free survival (PFS), and the frequency and degree of adverse events (AEs).
Results: Thirty patients (14 male; median age, 64 years) were enrolled. The RR was 13.3%, with a median follow-up time of 9.3 months. The median OS and PFS were 7.6 and 3.8 months, respectively. From the beginning of first-line treatment, the median OS and PFS were 14.2 and 9.3 months, respectively. Grade 3 or 4 AEs were seen in 70% of patients.
Zaibet et al (Brit J of Cancer) Published online: 29 January 2022:
Retrospective real-world multicenter study, from 2011 to 2019, patients with mPA receiving Gem-Nab (Gem 1000 mg/m² + Nab 125 mg/m², 3 out of 4 weeks) or Gem alone were included after progression on FFX.
Results: 427 patients included, patients receiving Gem-Nab had more metastatic sites, peritoneal disease and less PS 2 (24% vs. 35%). Gem-Nab was associated with better disease control rate (DCR) (56% vs. 32%; P < 0.001), progression-free survival (PFS) (3.5 vs. 2.3 months; 95% CI: 0.43–0.65) and overall survival (OS) (7.1 vs. 4.7 months; 95% CI: 0.53–0.86). Multivariate analysis, gem-Nab and PS 0/1 were associated with better OS and PFS. Grade 3/4 toxicity was more frequent with Gem-Nab (44% vs. 29%).
Effectiviteit
Primair eindpunt
Bijwerkingen (totaal/ gerelateerd aan de behandeling)
- Lethaal: 0 %
- Acuut, ernstig (graad 3-4): 37.5 %/ 70%
- Staken van de behandeling i.v.m. bijwerkingen: 12.5 %
Kwaliteit van leven
Kwaliteit van leven analyse
Beperkingen van de studie
Portal et al; Observationele case series
Mita et al; phase II studie met 30 patienten
Zaibet et al: retrospectieve real-world data. 427 patienten
Referentie(s)
Discussie / advies / wanneer herbeoordelen
De aanvraag betreft een verzoek tot indiening van een off-label indicatie voor de combinatie nabpaclitaxel/gemcitabine na progressie op FOLFIRINOX bij het gemetastaseerd pancreascarcinoom.
De aangeleverde studies zijn alle of retrospectief of betreffen kleine, niet gerandomiseerde fase 2 studies. Level of evidence IV-V.
CieOOM is van mening dat de groep patiënten met gemetastaseerd pancreascarcinoom met progressie na een eerstelijnsbehandeling groot genoeg is om een prospectieve en gerandomiseerde studie op te zetten.
CieOOM ziet op basis van deze argumenten geen indicatie voor een off-label aanvraag voor de combinatie gemcitabine/nabpaclitaxel te ondersteunen.
Datum besproken vergadering
04/10/2022